



Next: Coulomb Interactions in periodic
Up: Periodic Wave-Function
Previous: Periodic orbitals
Contents
The wave-function for high pressure hydrogen
In order to study the high pressure hydrogen we used the periodic generalization of the JAGP wave-function defined in the chapter 1. In the two-body 1.2.3 terms and one-body 1.2.2 terms the distances electron-electron and electron-ion are replaced with the periodic distance:
![$\displaystyle \left\vert \vec \xi_i - \vec \xi_j \right\vert = \frac{L}{ \pi } ...
...i_j^2 ) \right ] + \sin^2\left [ \frac{ \pi }{L } (\xi_i^3-\xi_j^3 ) \right ] }$](img417.png) |
(4.8) |
where 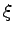 indicates electron and proton coordinates
indicates electron and proton coordinates 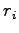 ,
,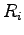 .
Both in the pairing determinant 1.2.1 and in the three-body Jastrow 1.2.4 we used one orbital per atom as basis set. In the first one we used periodic Gaussian orbitals 4.6 while in the second
.
Both in the pairing determinant 1.2.1 and in the three-body Jastrow 1.2.4 we used one orbital per atom as basis set. In the first one we used periodic Gaussian orbitals 4.6 while in the second 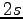 orbitals defined as:
orbitals defined as:
where the distance between electrons is defined as 4.8. We found that this basis set was sufficient to describe accurately the systems studied.




Next: Coulomb Interactions in periodic
Up: Periodic Wave-Function
Previous: Periodic orbitals
Contents
Claudio Attaccalite
2005-11-07
![$\displaystyle \left\vert \vec \xi_i - \vec \xi_j \right\vert = \frac{L}{ \pi } ...
...i_j^2 ) \right ] + \sin^2\left [ \frac{ \pi }{L } (\xi_i^3-\xi_j^3 ) \right ] }$](img417.png)